The gas laws provide a relationship between the absolute temperature of a gas, the pressure exerted by a gas on the walls of its container, the volume occupied by the gas, and the amount of gaseous substance. Boyle’s law is an important gas law that provides a relationship between pressure and volume. A brief explanation of Boyle’s law and its mathematical expression is provided in this article.

Boyle’s Law and its Expression
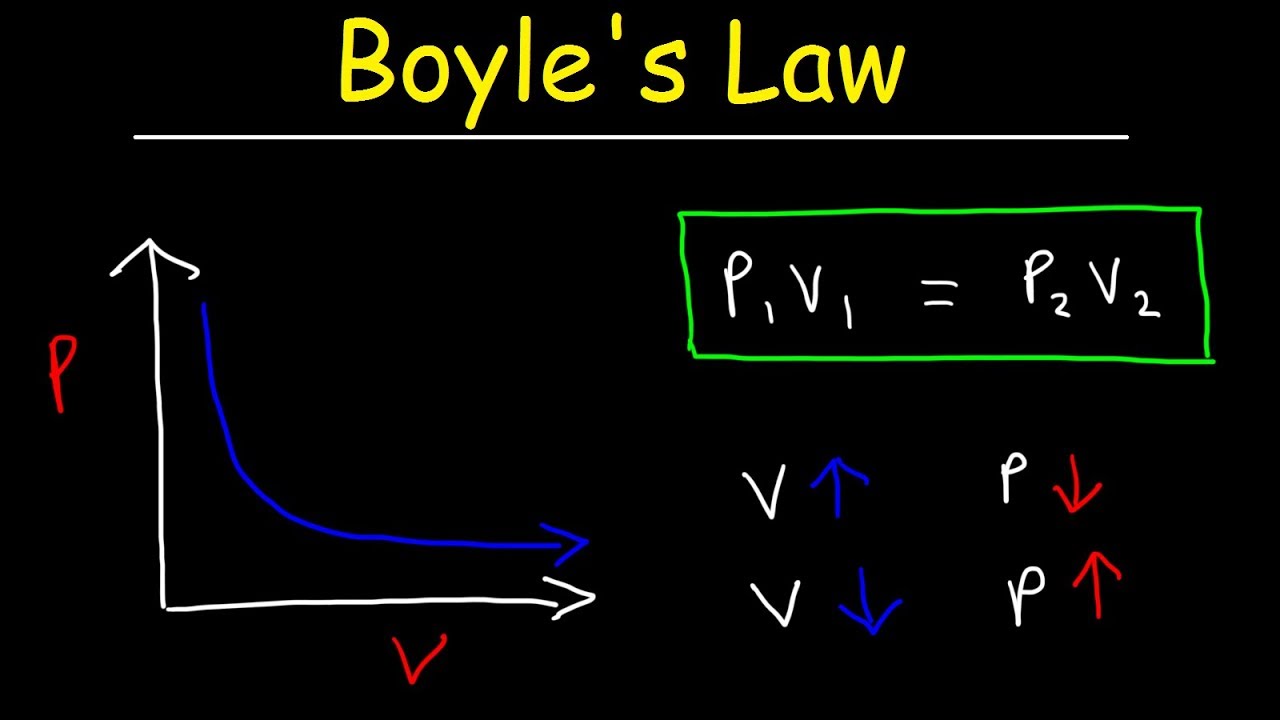
The Anglo-Irish scientist Robert Boyle formulated the relationship between the pressure exerted by a gas on the walls of its container and the volume occupied by the gas in the year 1662. Boyle’s law states that the pressure exerted by a gas is inversely proportional to the volume occupied by the gas when the temperature and amount of gaseous substance are kept constant).
To simplify – the smaller the volume occupied by the gas, the greater the force exerted by the gas on the container. Mathematically, this law can be expressed as follows:
P ∝ (1/V) (or) PV = k (at constant temperature and amount of gas).
Where:
- P is the pressure exerted by the gas.
- V is the volume occupied by the gas.
- k is a constant.
Another mathematical expression of this law is:
P1V1 = P2V2 (when the temperature and amount of gas are kept constant).
Where:
- P1is the initial pressure exerted by the gas.
- V1is the initial volume occupied by the gas.
- P2is the final pressure exerted by the gas.
- V2is the final volume occupied by the gas.
Solved Numerical on Boyle’s Law
A gas exerts 1 atmosphere of pressure on a 1-liter container. If the gas is completely transferred to a 2-liter container, what would be the pressure exerted by the gas on the walls of the 2-liter container? (assume that the absolute temperature of the gas is kept constant).
The initial pressure exerted by the gas (P1) is 1 atm. The initial volume occupied by the gas (V1) is 1 liter. The final volume occupied by the gas (V2) is 2 liters. Therefore, the final pressure exerted by the gas (P2) is [(1 L * 1 atm)/2 L] = 0.5 atmosphere.
To learn more about Boyle’s law and other important concepts in chemistry (such as chemical bonding and metallic bond), subscribe to the BYJU’S YouTube channel and enable notifications.